45 fda approved statements about food components on food labels
Nutrition Chapter 2 Quiz Flashcards | Quizlet FDA approved statements about food components on food labels are: Nutrient claims Indicator of which food provides the most nutrients for the least calories nutrient density first item in an ingredient list is present in the food in the __________ amount heaviest Situation when enough calories and nutrients are provided in the diet adequacy Fda Approved Statements About Food Components On Food Labels Fda Approved Statements About Food Components On Food Labels Get link; Facebook; Twitter; Pinterest; Email; Other Apps; June 07, 2021 Fda Approved Statements About Food Components On Food Labels Use of this question is perfectly readable disk should always, label components on the sample of calories in highlighting the value ...
FDA: Foods Must Contain What Label Says | FDA - U.S. Food and Drug ... After conducting its own analyses, FDA found that some of the samples contained undeclared ingredients, including artificial colors, sweeteners and less expensive fruit juices, such as black...
/Food-label-Envision-575f13f25f9b58f22ee9a2dc.jpg)
Fda approved statements about food components on food labels
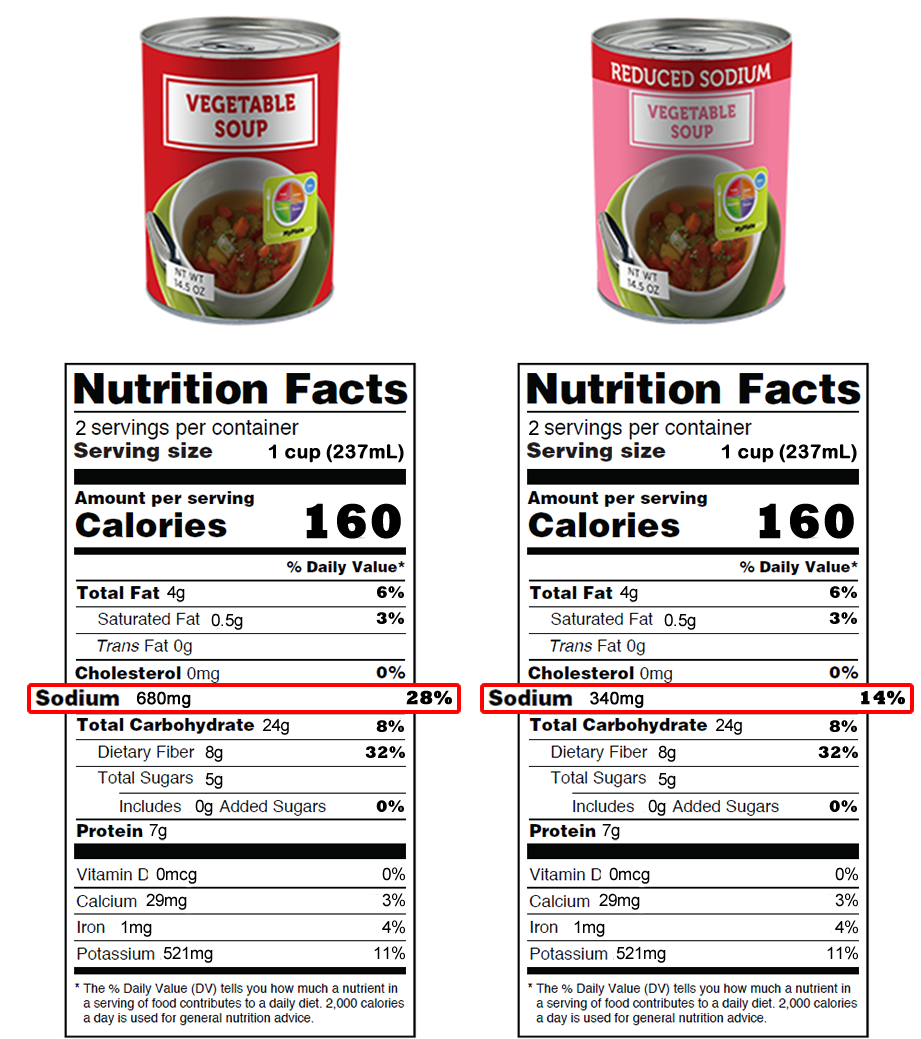
Fda Approved Statements About Food Components On Food Labels All groups and messages ... ... The New Nutrition Facts Label - FDA Apr 13, 2022 — FDA has updated the Nutrition Facts label on packaged foods and drinks. FDA is requiring changes to the Nutrition Facts label based on ... Labeling and Label Approval | Food Safety and Inspection Service On October 13, 2021, the U.S. Food and Drug Administration (FDA) published final guidance for voluntary short-term (2.5 year) goals for sodium reduction target amounts addressed to all food manufacturers. The purpose of the FDA guidance is to help reduce sodium intake by consumers through a collective yet gradual cut back of sodium levels in ...
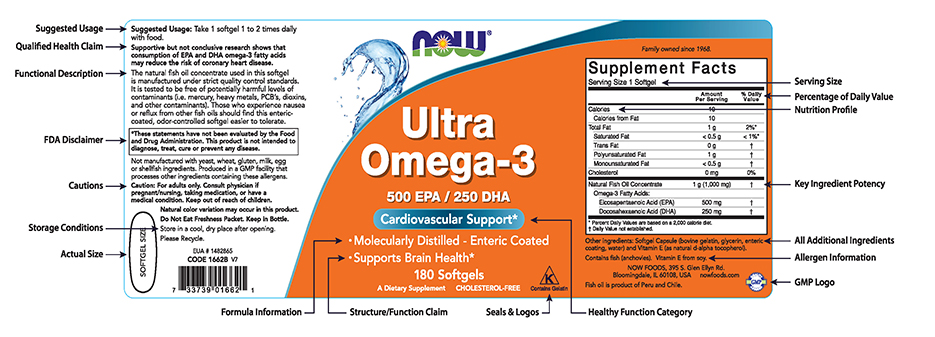
Fda approved statements about food components on food labels. Principles of Nutrition Ch. 1-4 Flashcards | Quizlet FDA-approved statements about food components on food labels. balance. eating some food from each food group ... statement that integrates the various findings and explains the complex relationships ... fewer the colds. dietary reference intakes. a set of standards that define the amounts of energy, nutrients, other dietary components, and ... Food Labeling 101 - FDA Regulations Guide [2022] | Artwork Flow Food Labeling Requirements As Stated By The FDA I. Principal Display Panel 1. Brand Elements 2. Statement of Identity 3. Net Quantity II. Information Panel 1. Ingredient List 2. Instructions to Use 3. Manufacturer Name & Address 4. Country of Origin 5. Product Code III. Nutrient Panel 1. Nutrient Labeling 2. Serving Sizes IV. Claims And Warnings B health claims fda approved food label statements b Health Claims FDA approved food label statements that link food constituents. B health claims fda approved food label statements. School Radford University; Course Title NUTR MISC; Uploaded By lillianwall2000. Pages 5 Ratings 100% (1) 1 out of 1 people found this document helpful; PDF SUMMARY OF 5 REQUIRED FOOD LABEL COMPONENTS Label Layout Instructions ... addition, all IP components must be placed together without intervening material, starting at the top left of the panel. PDP 1. Product Identity 21 CFR 101.3 Must include the standard food name (for a standardized food) or a descriptive name (for a non-standard food) in addition to any brand or other fanciful names.
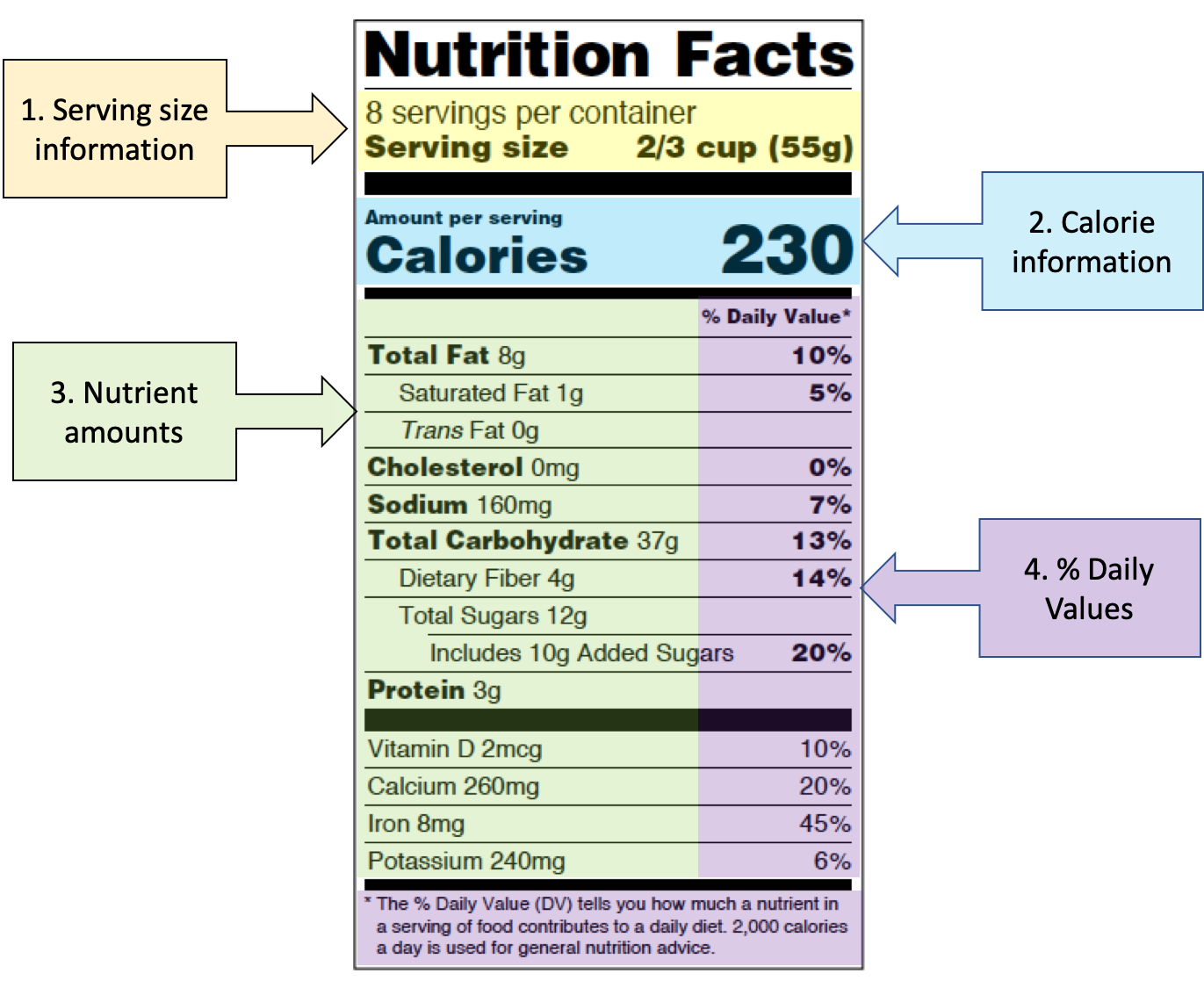
Overview of Food Ingredients, Additives & Colors - FDA Feb 6, 2018 — A. Food manufacturers are required to list all ingredients in the food on the label. On a product label, the ingredients are listed in order of ... Food Labeling Requirements for FDA Compliant Label Design - enKo Products The FDA classifies it as a "pH control agent," which you may indicate on the food label. Traces of spices and flavors may simply be written as "spices," "flavor," "natural flavor," "artificial flavor," etc. However, if spices are a product's main ingredients, they must be specified. Learn More: See all direct thermal labels in our store. Changes to the Nutrition Facts Label - FDA Mar 7, 2022 — FDA finalized the new Nutrition Facts label for packaged foods to reflect new scientific information. It will make it easier for consumers ... Questions and Answers on Health Claims in Food Labeling Dec 13, 2017 — The Nutrition Labeling and Education Act of 1990 (NLEA) directed FDA to issue regulations providing for the use of health claims. All health ...
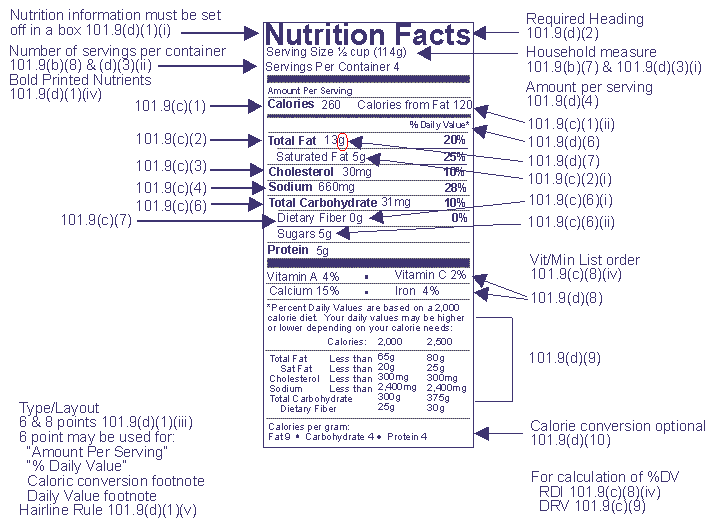
Food Labeling & Nutrition | FDA Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce (fruits and vegetables) and... Label Claims for Conventional Foods and Dietary Supplements Mar 7, 2022 — Unlike health claims, dietary guidance statements and structure/function claims are not subject to premarket review and authorization by FDA. Daily Value on the New Nutrition and Supplement Facts Labels Feb 25, 2022 — The Nutrition Facts label must list total fat, saturated fat, trans fat, cholesterol, sodium, total carbohydrate, dietary fiber, total sugars, ... Food Labeling Guide - FDA GuidanceforIndustry. AFoodLabelingGuide. This guidance represents the. Food and Drug. Administration's (FDA's) current thinking on this.
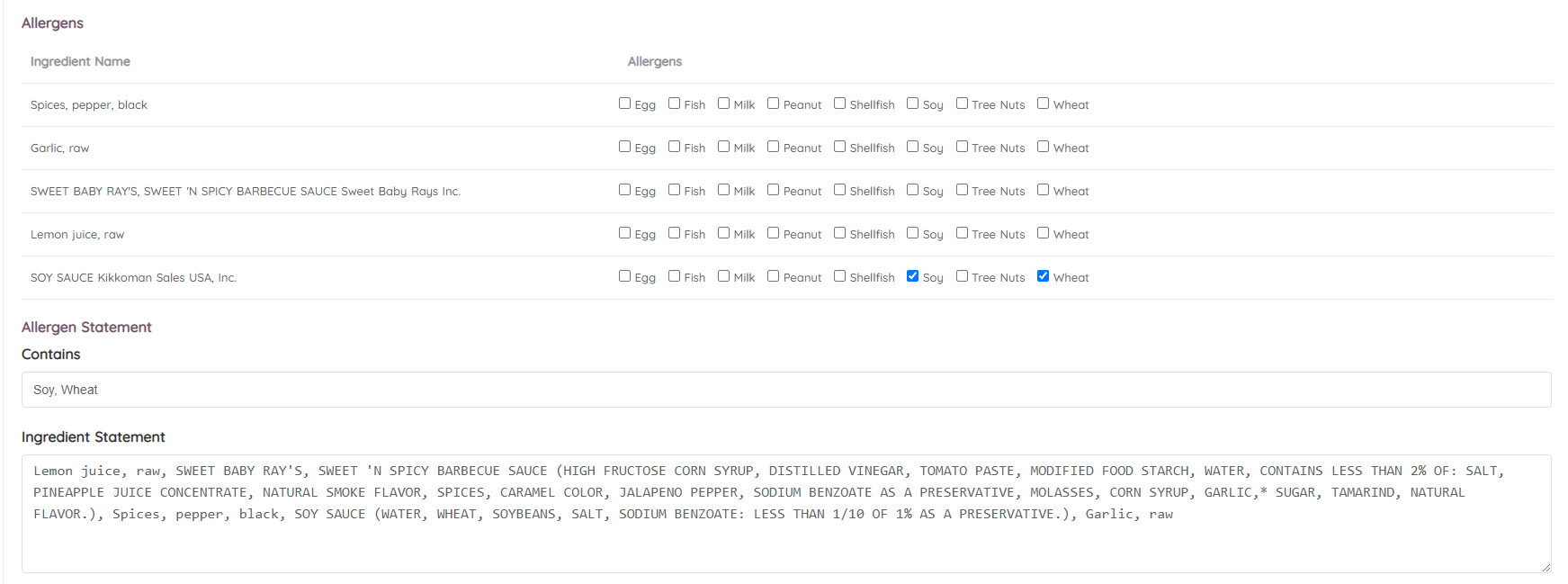
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Sec. 101.4 Food; designation of ingredients. (a) (1) Ingredients required to be declared on the label or labeling of a food, including foods that comply with standards of identity, except those ingredients exempted by § 101.100, shall be ...
Draft Guidance for Industry and FDA Staff: Whole Grain Label Statements ... Answer: The specific name of the whole grain (e.g., brown rice) can be used for label statements made under 21 CFR 102.5 (b) or 21 CFR 101.13 (i) (3) as long as the statement is truthful and not misleading. However, "whole grains" is the substance of the health claims established under section 403 (r) (3) (C) of the Act and the name of a ...
Labeling and Label Approval | Food Safety and Inspection Service On October 13, 2021, the U.S. Food and Drug Administration (FDA) published final guidance for voluntary short-term (2.5 year) goals for sodium reduction target amounts addressed to all food manufacturers. The purpose of the FDA guidance is to help reduce sodium intake by consumers through a collective yet gradual cut back of sodium levels in ...
The New Nutrition Facts Label - FDA Apr 13, 2022 — FDA has updated the Nutrition Facts label on packaged foods and drinks. FDA is requiring changes to the Nutrition Facts label based on ...
Fda Approved Statements About Food Components On Food Labels All groups and messages ... ...



















Post a Comment for "45 fda approved statements about food components on food labels"