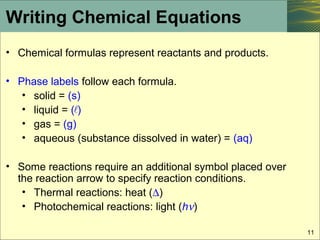
39 phase labels in chemical equations
Lagrangian mechanics - Wikipedia applies to each particle. For an N particle system in 3 dimensions, there are 3N second order ordinary differential equations in the positions of the particles to solve for.. The Lagrangian. Instead of forces, Lagrangian mechanics uses the energies in the system. The central quantity of Lagrangian mechanics is the Lagrangian, a function which summarizes the dynamics of … Chemical Reactions and Equations - Lardbucket.org Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). ... According to the solubility rules, Ca 3 (PO 4) 2 is insoluble, so it has an (s) phase label. To balance this equation, we need two phosphate ions and three calcium ions; we end ...
Phase Labels - YouTube Clark College Tutoring and Writing Center tutors Kevin Martin and Joey Smokey explain the usage of phase labels in chemical reactions such as (s), (l), (g), ...

Phase labels in chemical equations
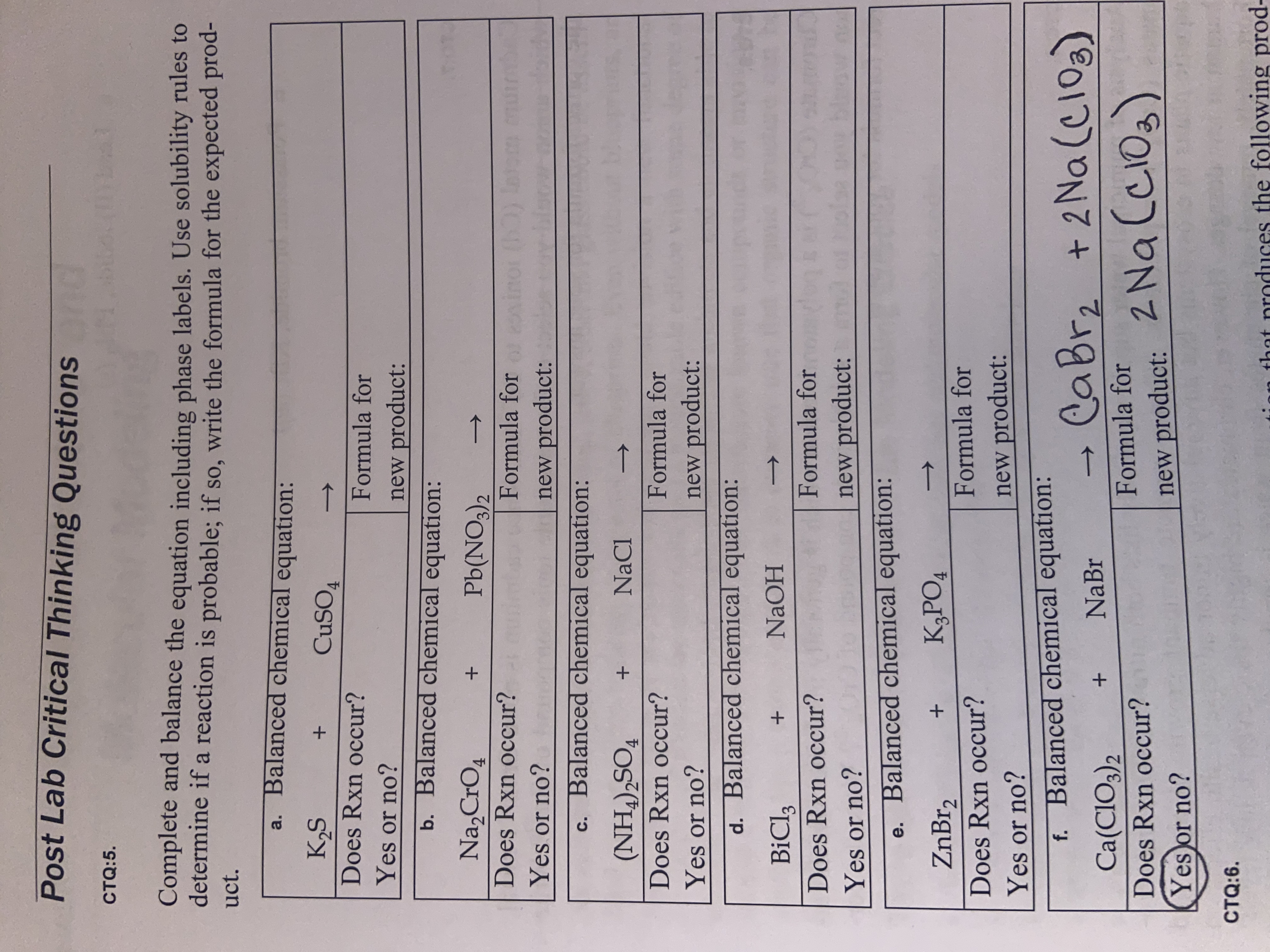
Definition of dissolve and the state label (aq) in chemical equations C O X 2 ( a q) would imply dissolved carbon dioxide in water (i.e.,to our eyes it is a single phase, just like an unopened bottle of coke). B r X 2 ( a q) would imply homogeneous solution of liquid bromine in water. N a C l ( a q): Salt dissolved in water. A colloid is a heterogeneous phase. Many chemical equations also include phase labels for Many chemical equations also include phase labels for the substances s for solid from AGRICULTUR 847,272 at Eastern Visayas State University - Tacloban City Main Campus Solved Write the chemical equation (including phase labels) | Chegg.com Transcribed image text: Write the chemical equation (including phase labels) and balance the following word equation Potassium carbonate solution and chromium (III) chloride solution produce a precipitate of chromium (III) carbonate and aqueous potassium chloride. ce You do not need to write the formula using subscripts just put next to symbol for example Na2Cr204 (aq) HTML Editores BIŲ A- A ...
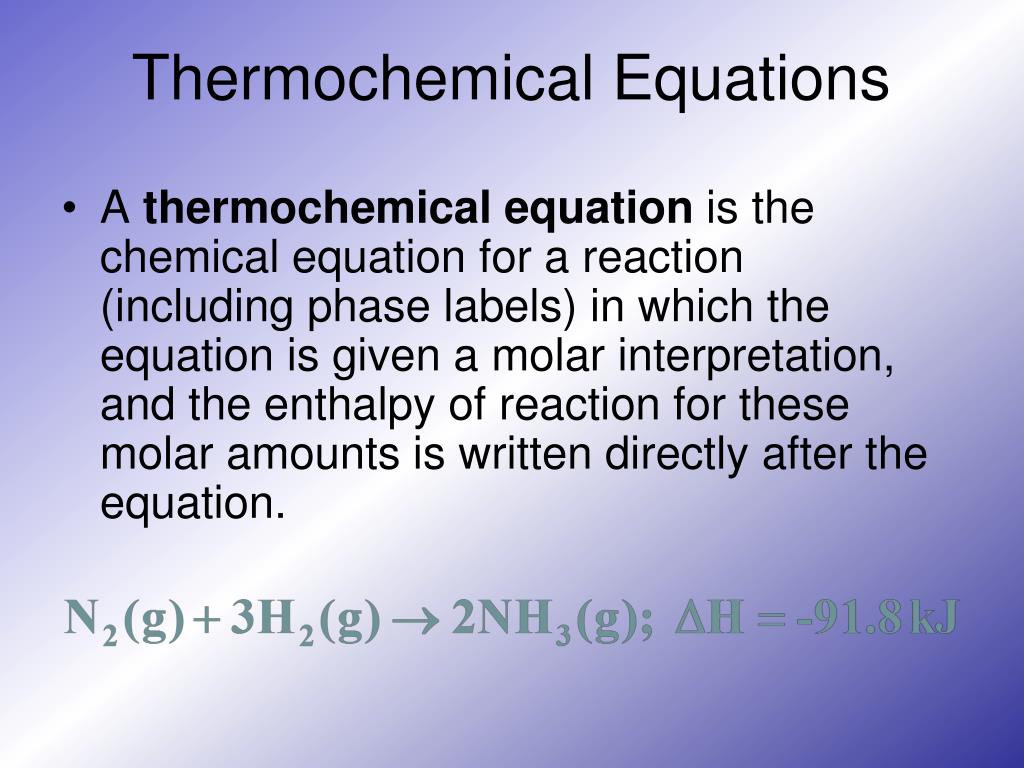
Phase labels in chemical equations. Analyzing Learned Molecular Representations for Property … Advancements in neural machinery have led to a wide range of algorithmic solutions for molecular property prediction. Two classes of models in particular have yielded promising results: neural networks applied to computed molecular fingerprints or expert-crafted descriptors and graph convolutional neural networks that construct a learned molecular representation by operating … The Chemical Equation - Introductory Chemistry - 1st Canadian Edition Write and balance the chemical equation that represents nitrogen and hydrogen reacting to produce ammonia, NH 3. Answer N 2 + 3H 2 → 2NH 3 Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Chapter 5 - Chemical Reactions and Equations - CHE 105/110 ... Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). ... phase label. To balance this equation, we need two phosphate ions and three calcium ions; we end up with six water molecules to balance the equation: 2H 3 PO 4 (aq) + 3Ca(OH) 2 ... SOLVED:16. Write a balanced equation (including phase labels) for the ... we are just looking to balance some chemical equations. So the first one we have a gcl in the solid state. I won't list. I went right down the states but I will say them aloud just to save a bit of times what a gcl solid at two and H. Three A quits. That gives us A G. And H. 32 plus acquis Art C. L minus liquids. The next example we've got C R E N two cl two cl acquits R two H 20.
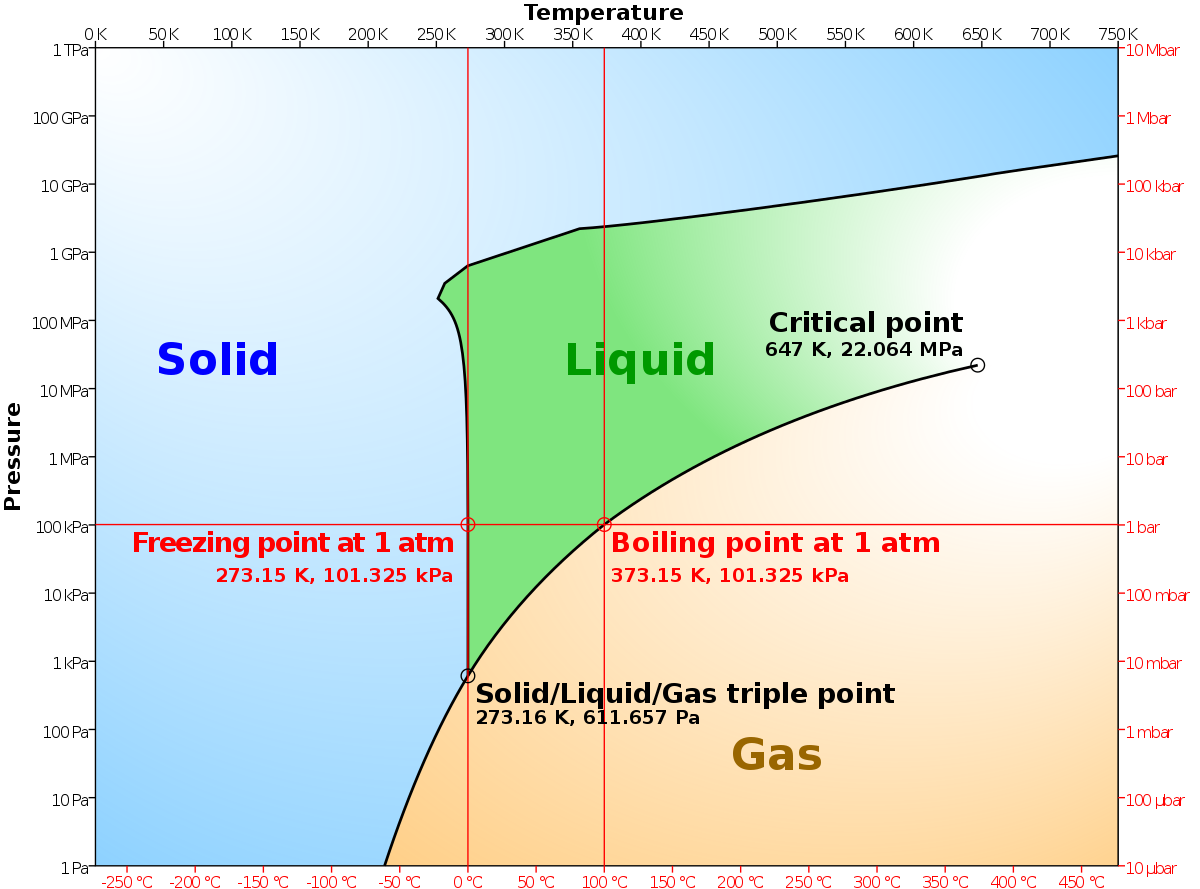
End-of-Chapter Material Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4Na (s) + 2Cl2(g) → 4NaCl (s) should not be considered a proper chemical equation. Explain why H2(g) + 1/2O2(g) → H2O (ℓ) should not be considered a proper chemical equation. Structures, Phase Transitions and Tricritical Behavior of the … Oct 21, 2016 · We have examined the crystal structures and structural phase transitions of the deuterated, partially deuterated and hydrogenous organic-inorganic hybrid perovskite methyl ammonium lead iodide ... State symbols and phase changes | StudyPug The phase can affect how reactive a substance is, but changing phase (a physical change) is not the same as changing the substance (a chemical change). A fully detailed chemical equation will show the state (or phase) of matter that the atoms or molecules are in. These states are: Solid, given the symbol (s) Liquid, given the symbol (l) Arc flash - Wikipedia An arc flash is the light and heat produced from an electric arc supplied with sufficient electrical energy to cause substantial damage, harm, fire, or injury. Electrical arcs experience negative incremental resistance, which causes the electrical resistance to decrease as the arc temperature increases. Therefore, as the arc develops and gets hotter the resistance drops, drawing more …
EEP - Electrical Engineering Portal | Energy and Power For All Sep 19, 2022 · Procedures and activities during the design and tendering phase of HV project engineering. Electric Motor 19 Essential Information You Can Find On Motor Nameplate. Motor nameplate is normally located on all produced electric motors. Understanding nameplate information can be hard sometimes, but is essential. In most countries it is a ... chemical equation | Yeah Chemistry Formulas of the reactants appear on the left side of the equation; those of products are written on the right. In many cases it is useful to indicate the state or phases of the substance in an equation.You use the following phase labels: (g) = gas , (l) = liquid , (s) = solid , (aq) = water solution End-of-Chapter Material - Introductory Chemistry - 1st Canadian Edition Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4Na(s) + 2Cl 2 (g) → 4NaCl(s) should not be considered a proper chemical equation. Explain why H 2 (g) + ½O 2 (g) → H 2 O(ℓ) should not be considered a proper chemical ... Homeschool Printables & Downloads - Five J's Homeschool This 8-pg PDF worksheet set covers the four states of matter: solids, liquids, gases, and plasma. It includes a color chart of the different states of matter, included labels for the changes between each state, the same chart with the labels removed so that the student can fill in the appropriate terms, an answer key for the fill-in-the-blank worksheet, and a word list of all the terms related ...
Regional Screening Levels (RSLs) - What's New | US EPA May 18, 2022 · The labels for adult and child body weight in the Calculator output were corrected. The target risk labels in the Resident Air Supporting Table were corrected. The Inhalation Unit Risk for TCDD was changed to 38 and the Generic Tables were updated. For assistance/questions please use the Regional Screening Levels (RSLs) contact us page. For ...
End-of-Chapter Material - Introductory Chemistry- 1st Canadian Edition Write a chemical reaction for the boiling of water, including the proper phase labels. Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why. 4 Na(s) + 2 Cl 2 (g) → 4 NaCl(s)
Properties of the density functional response kernels and its ... Sep 15, 2022 · The two first order derivatives, the electronic density [ρ(r) = (δE/δv(r)) N] and the electronic chemical potential [μ = (∂E/∂N) v], have been studied intensively as well as two of the second order derivatives, the chemical hardness [η = (∂ 2 E / ∂ N 2) v] and the Fukui function [f(r) = δ 2 E/∂Nδv(r)].The third one, the linear response kernel χ(r, r′) [= (δ 2 E / δ v (r ...
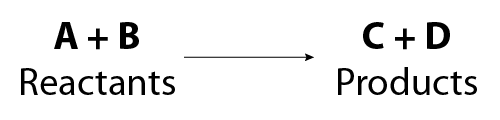
What are Chemical Equations? Detailed Explanation, Examples - BYJUS An example of an ionic chemical equation is provided below. Chemical Equation: CaCl 2 + 2AgNO 3 → Ca (NO 3) 2 + 2AgCl↓. Ionic Equation: Ca 2+ + 2Cl - + 2Ag + + 2NO 3- → Ca 2+ + 2NO 3- + 2AgCl↓. Comparing the reactants and the products of the ionic equation and the chemical equation, it can be observed that the Ca 2+ ( calcium ion ...
Solved 1. Write balanced chemical equations using proper | Chegg.com 1. Write balanced chemical equations using proper phase labels for these reactions: (you may also handwrite in the equations) a) Iron metal with oxygen gas to produce solid iron (III) oxide, also known as rust.
Optical Metasurfaces for Energy Conversion | Chemical Reviews Nanostructured surfaces with designed optical functionalities, such as metasurfaces, allow efficient harvesting of light at the nanoscale, enhancing light–matter interactions for a wide variety of material combinations. Exploiting light-driven matter excitations in these artificial materials opens up a new dimension in the conversion and management of energy at the nanoscale. In …
EXAM #2: Chapter 4 Flashcards | Quizlet Write a chemical reaction for the boiling of water, including the proper phase labels. ... 2. Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. ... 3. Explain why 4Na (s)+2ClX2 (g) 4NaCl (s) should not be considered a proper chemical equation.
Solved Write the chemical equation (including phase labels) | Chegg.com Transcribed image text: Write the chemical equation (including phase labels) and balance the following word equation Potassium carbonate solution and chromium (III) chloride solution produce a precipitate of chromium (III) carbonate and aqueous potassium chloride. ce You do not need to write the formula using subscripts just put next to symbol for example Na2Cr204 (aq) HTML Editores BIŲ A- A ...
Many chemical equations also include phase labels for Many chemical equations also include phase labels for the substances s for solid from AGRICULTUR 847,272 at Eastern Visayas State University - Tacloban City Main Campus
Definition of dissolve and the state label (aq) in chemical equations C O X 2 ( a q) would imply dissolved carbon dioxide in water (i.e.,to our eyes it is a single phase, just like an unopened bottle of coke). B r X 2 ( a q) would imply homogeneous solution of liquid bromine in water. N a C l ( a q): Salt dissolved in water. A colloid is a heterogeneous phase.







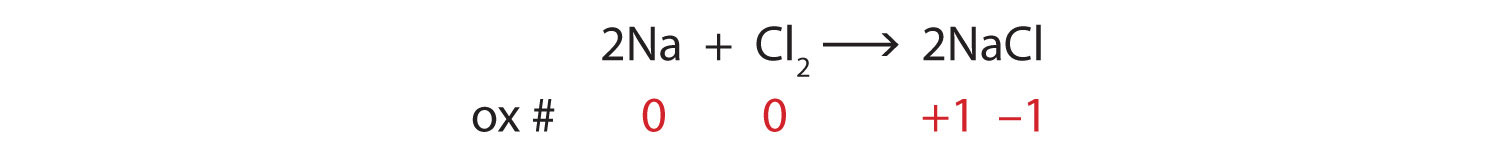


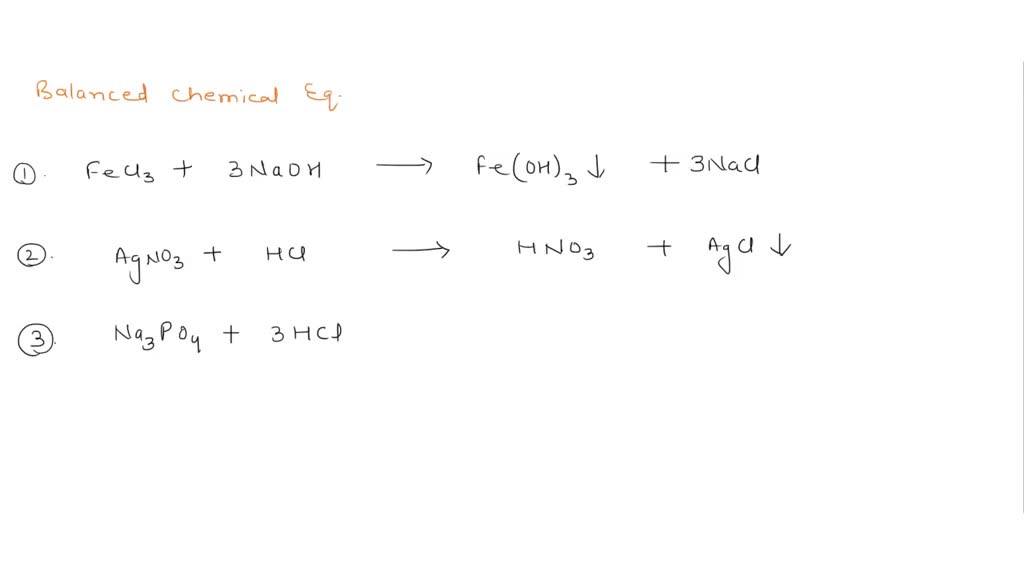
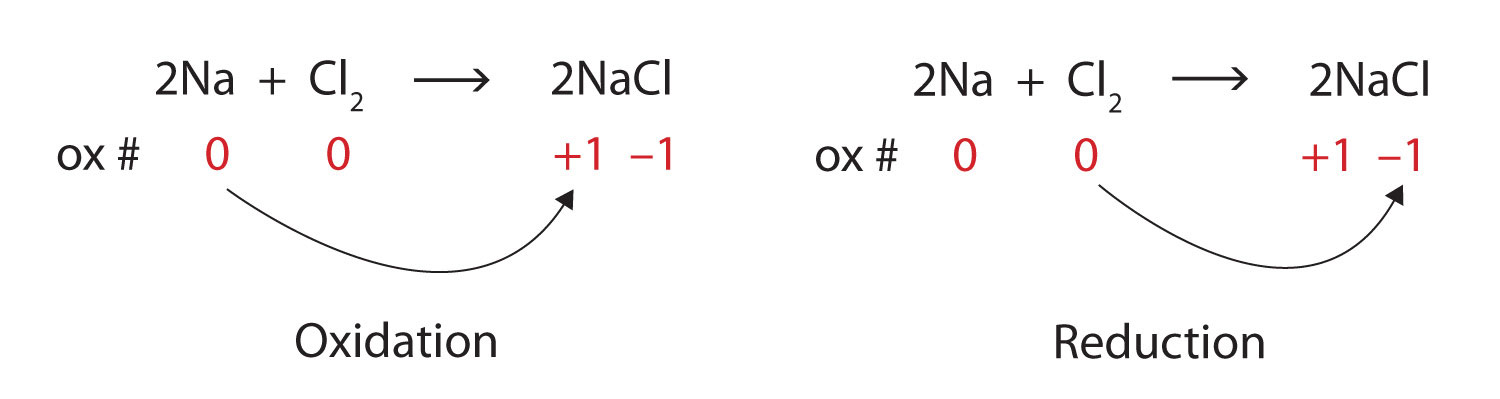






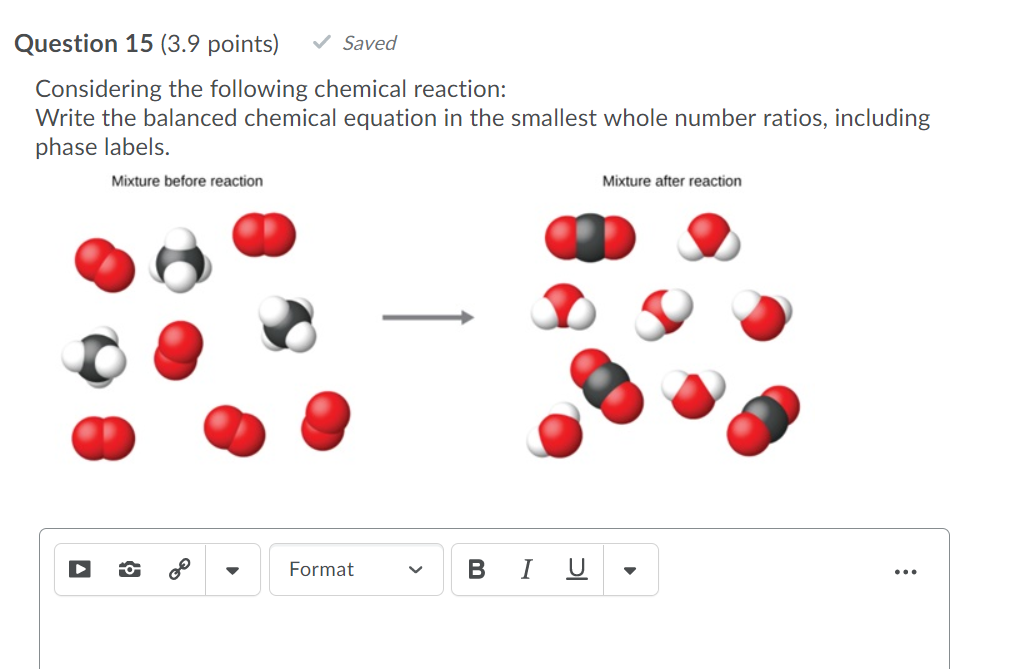
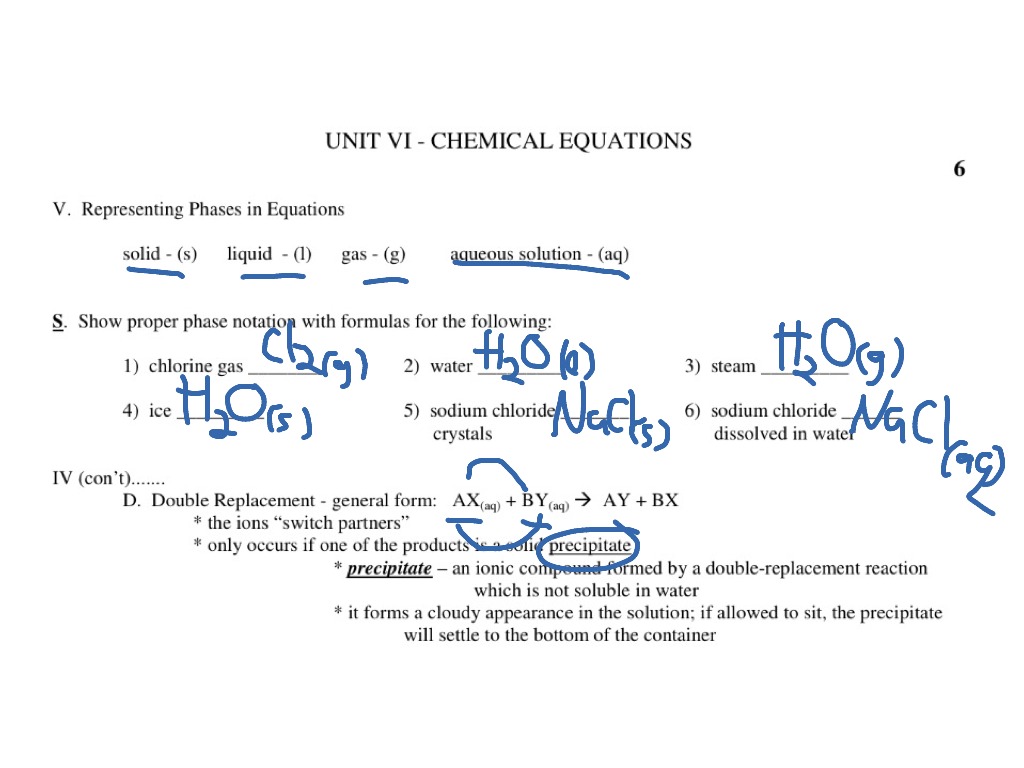


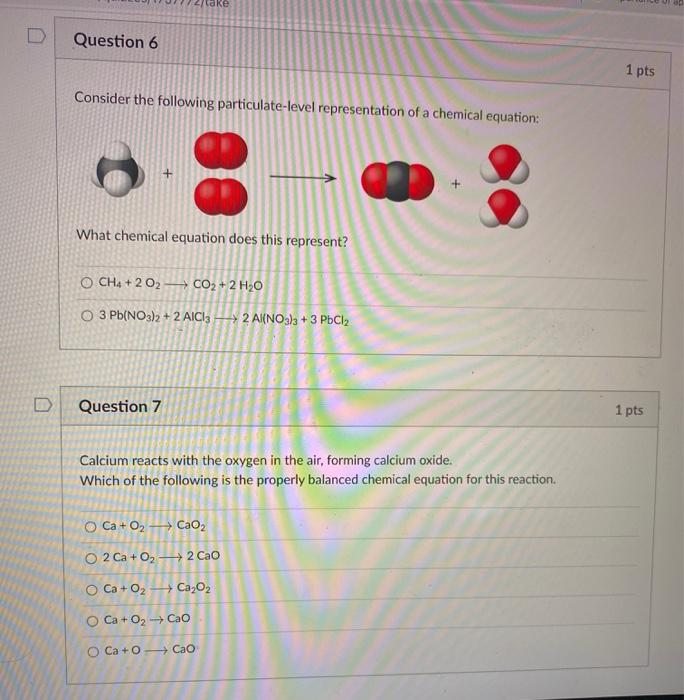



Post a Comment for "39 phase labels in chemical equations"